


For example, hydrogen has one electron in the s-orbital of the first shell, so its configuration is written 1s 1. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. The numbers of electrons that can occupy each shell and each subshell arise from the equations of quantum mechanics, in particular the Pauli exclusion principle, which states that no two electrons in the same atom can have the same values of the four quantum numbers. This gives two electrons in an s subshell, six electrons in a p subshell, ten electrons in a d subshell and fourteen electrons in an f subshell. The maximum number of electrons that can be placed in a subshell is given by 2(2 l + 1). For example, the 3d subshell has n = 3 and l = 2. The values l = 0, 1, 2, 3 correspond to the s, p, d, and f labels, respectively. The value of l is in the range from 0 to n − 1. The factor of two arises because the allowed states are doubled due to electron spin-each atomic orbital admits up to two otherwise identical electrons with opposite spin, one with a spin + 1⁄ 2 (usually denoted by an up-arrow) and one with a spin of − 1⁄ 2 (with a down-arrow).Ī subshell is the set of states defined by a common azimuthal quantum number, l, within a shell. For example, the first shell can accommodate 2 electrons, the second shell 8 electrons, the third shell 18 electrons and so on. An atom's nth electron shell can accommodate 2 n 2 electrons. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.Įlectron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.Īn electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. This is also useful for describing the chemical bonds that hold atoms together. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements.

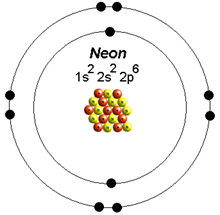
Mathematically, configurations are described by Slater determinants or configuration state functions.Īccording to the laws of quantum mechanics, for systems with only one electron, a level of energy is associated with each electron configuration and in certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively.Įlectronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.


 0 kommentar(er)
0 kommentar(er)
